INTRODUCTION
Although gastrectomy is considered the most effective treatment for potentially curable gastric cancer, its role is limited by the high morbidity and mortality following resection. Therefore, novel biomarkers that accurately predict recurrence and survival could provide clinicians with useful preoperative information to improve surgical outcomes.
The tumor-node-metastasis (TNM) staging system is considered the gold standard for prognostication in malignant tumors [
1]. However, variable outcomes from the same tumor stage, and the inability to incorporate other variables are drawbacks [
1,
2]. Given the imperfections of the TNM system and dissatisfaction with biomarkers developed so far, more research is needed to establish simple but accurate new biomarkers.
Computed tomography (CT) scans has been considered the standard for evaluating the body composition, including muscle mass (i.e., muscle quantity) and muscle fat infiltration (i.e., muscle quality) [
3]. Abdominal CT scans are most commonly preferred for body composition analysis [
4], and the 3rd lumbar spine is considered the most common indicator [
5]. The most common location for analysis is the total abdominal wall musculature (i.e., erector spinae, ES; multifidus, MF; quadratus lumborum, QL; psoas, PS. external obliques, internal obliques, transversus abdominis, and rectus abdominis), followed by the paraspinal muscles (i.e., ES, MF, QL, PS muscle).
Loss of muscle mass has a significant impact on cancer outcomes, including survival of cancer patients [
6]. In a study by Shachar et al., low skeletal muscle index (SMI) was associated with worse survival in patients with gastrointestinal malignancies (hazard ratio, HR; 1.5 for gastroesophageal cancer and HR 2.2 for colorectal cancer) [
7]. Similarly, in a study by Kuwada et al. [
8], low preoperative SMI was a predictor of overall survival (OS) in most studies of gastric cancer. However, in Hacker et al.ãs studies involving gastric and gastroesophageal junction cancer, SMI was not a predictor of [
9]. Therefore, prognostic role of SMI in gastrointestinal malignancies is unclear.
Muscle tissue usually contains only small amounts of fat, and when fat accumulates in excess, it is called myosteatosis. Advances in CT imaging techniques have enabled detailed analysis of muscle quality using muscle radiation attenuation (MRA) [
10-
12]. It has been reported that gastrointestinal cancer patients with myosteatosis have an increased mortality rate compared to those without [
13,
14].
The paraspinal muscles, stabilize the motion of spinal column, and the cross-sectional area of paraspinal muscle area (PMA) is related to the muscleãs ability to generate force. Several studies have reported the clinical role of the height-square-adjusted PMA, the paraspinal muscle index (PMI), in determining the survival of gastrointestinal malignancies [
9,
15-
17]. In the study of Hacker et al. [
9], PMI of ES/MF/QL muscles was a predictor of OS, whereas PMI of PS muscles was not. In our previous studies, PMI of ES/MF/QL/PS muscles was not a predictor of OS [
16,
17]. Therefore, the clinical role of PMI in gastrointestinal malignancies is unclear as consensus has not been established. With respect to MRA of the paraspinal muscles (PMRA), few studies have investigated the clinical value of PMRA in patients with gastrointestinal malignancies [
15-
17]. In our previous studies, preoperative PMRA of ES/MF/QL/PS muscles was an important determinant of OS and disease-free survival (DFS) in gastric cancer patients [
16,
17]. However, further studies are needed to draw conclusions about the clinical significance of PMRA as a predictor of survival.
The ES/MF muscles are the posterior components of the paraspinal muscles. While the ES muscles are the major component of the paraspinal muscles and act as global mobilizers, the MF muscles act as local stabilizers. Compared to psoas muscle, which are rich in type II muscle fibers, the ES/MF muscles are primarily composed of type I muscle fibers, which are characterized by slower contractile rates and higher lipid content than Type II fibers [
18]. Therefore, the body composition of the ES/MF muscle and its clinical significance may differ from that of the psoas muscle. In a study by Dohzono et al. [
15], PMRA of ES/MF muscle (PMRA
EM) was an important determinant of survival, whereas PMI of ES/MF muscle (PMI
EM) was not. However, the clinical significance of PMI
EM and PMRA
EM in gastric cancer is unclear, as only advanced gastrointestinal malignancies, including 24% of gastric cancer, were included in the study [
4].
Therefore, the purpose of this study is to investigate whether PMIEM and PMRAEM can predict the survival rate in patients with stage I to III gastric cancer that can be treated with gastrectomy. When PMIEM and PMRAEM are found to accurately predict patient recurrence and survival, their results can provide clinicians with useful preoperative information to improve surgical outcomes.
DISCUSSION
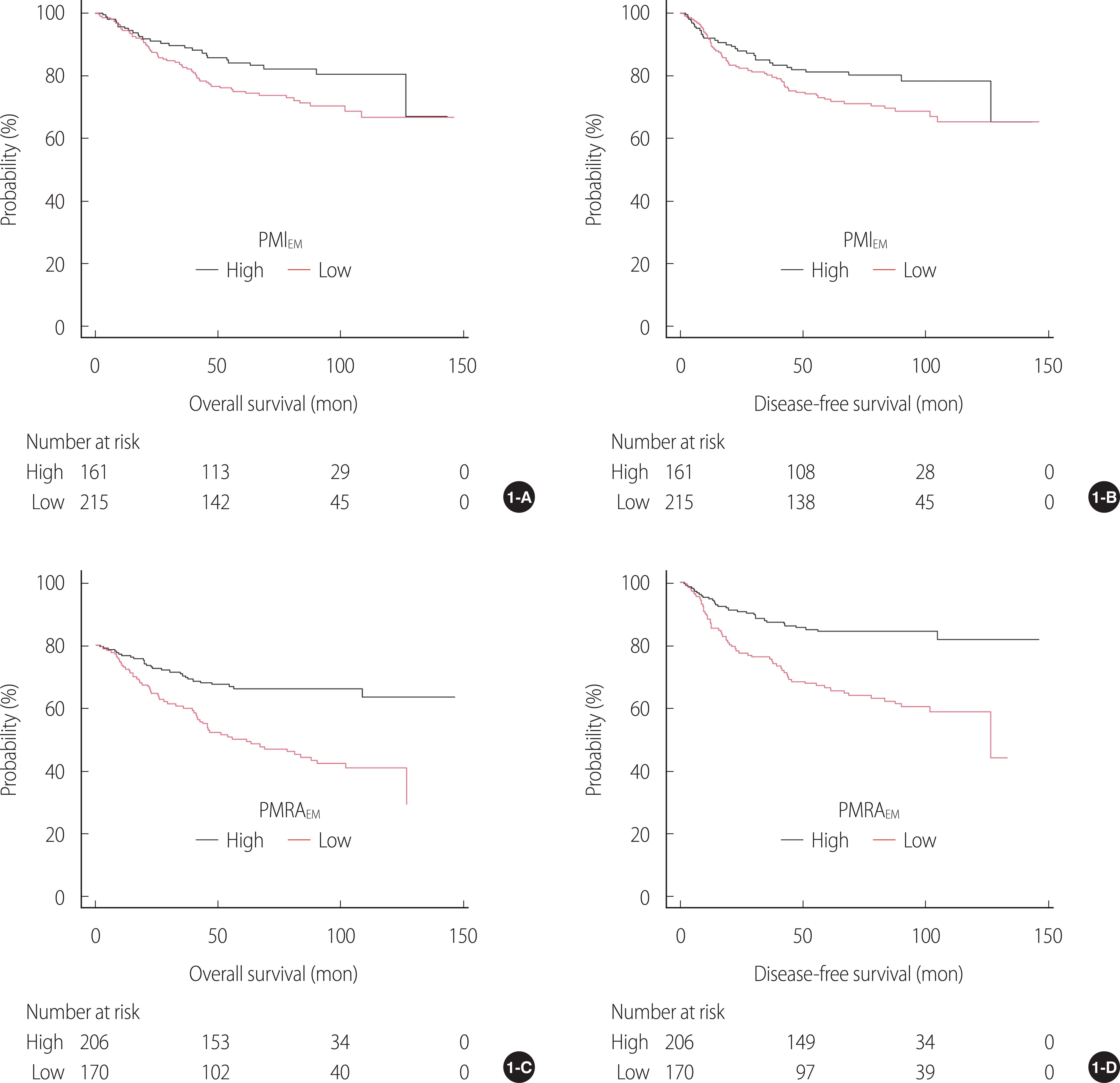
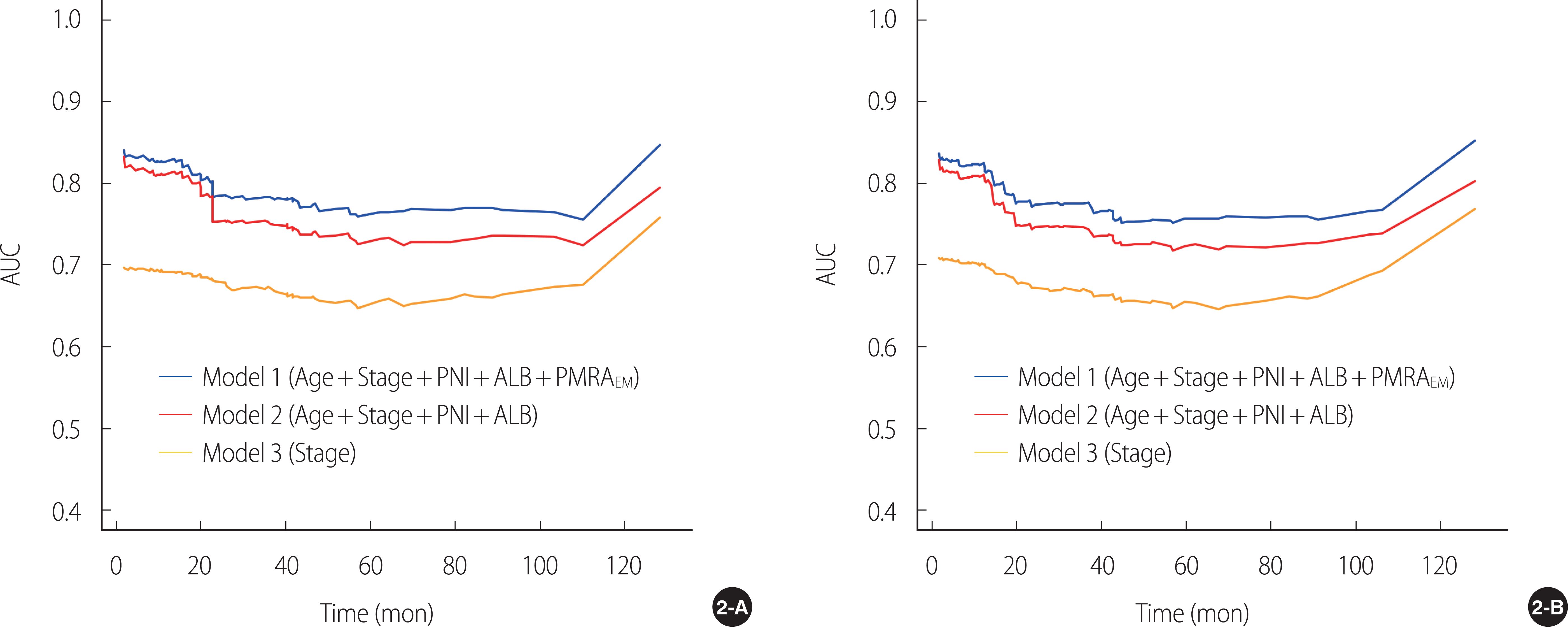
In the present study, PMRAEM was an independent predictor of survival. The five covariates derived from multivariate Cox regression analysis (i.e., PMRAEM along with age, TNM stage, perineural invasion, and serum albumin level) constituted Model 1. The iAUC and c-index of Model 1 for OS and DFS were higher than those of the nested models (i.e., Model 2 or Model 3).
CT scans has been considered the standard for evaluating the body composition. Because CT scans are a key imaging test for assessing and monitoring cancer staging and response in most cancer patients, studies of body composition determination in cancer patients have the advantage of avoiding additional radiation exposure [
3]. For analysis of body composition using CT scan, abdominal CT scans are most commonly preferred because of the relatively large amount of image data available for retrospective review [
4]. L3 levels in the abdomen are the most attractive landmarks for study [
5]. The most frequent regions of interest in the abdomen have been the total abdominal wall musculature followed by paraspinal muscles.
Muscle tissue usually contains only small amounts of fat, and when fat accumulates in excess, it is called myosteatosis. The gold standard for analyzing muscle adipose tissue infiltration is the biopsy. However, advances in CT imaging have enabled increasingly detailed analysis of muscle qualities using MRA in HU [
10-
12]. For measuring MRA, however, a specialized software to analyze CT images is required and can be expensive. In addition, measurement training is required as most software is not fully automatic. Finally, cutoff points to define low MRA are different according to gender, and they are diverse depending on the studies.
Recently, the clinical significance of muscle fat content in malignancies has been reported in the form of a systemic review. In a study by Aleixo et al., cancer patients with myosteatosis had an increased mortality (HR 1.75) compared to cancer patients without [
14]. In another study limited to gastrointestinal tumors, only patients with esophageal gastric cancer, cholangiocarcinoma, pancreatic cancer, and colorectal cancer had a higher mortality rate in patients with myosteatosis than in patients without myosteatosis [
13]. These findings, like ours, underscore the clinical importance of muscle fat content in gastric cancer.
In our previous reports, we also found that MRA of paraspinal muscles were significant prognostic factor for survival in patient with gastric cancer undergoing curative gastrectomy [
16,
17]. In particular, the c-index of the model including only paraspinal muscles was greater than the model including the total abdominal wall musculature. Considering that the paraspinal muscles are a small component of the total abdominal wall musculature, this result may give hope that the measurement time can be saved without compromising the accuracy of the measurement [
17].
These previous findings raised the question of whether body composition localized to the ES/MF muscle, a small component of the paraspinal muscle composed of type I muscle fibers, could be a radiological indicator of survival [
18]. Accordingly, we carried out this study, which measures body composition localized to ES/MF muscles at the L3 level.
Using the multivariate Cox model, patients with high PMRA
EM had a significantly lower risk in terms of OS (HR 0.48) and DFS (HR 0.49) compared to patients with low PMRA
EM, highlighting the clinical significance of PMRA
EM. In this study, while PMRA
EM was an important determinant of survival, PMIEM was not, which is consistent with a previous study by Dohzono et al. [
15], although there are some differences between them, including cutoffs, histology (gastric cancer vs. gastrointestinal malignancies including gastric cancer at 24%), and tumor stage (stage I-III vs. advanced stage). Therefore, in gastrointestinal malignancies, muscle quality (i.e., PMRA
EM) rather than muscle mass (i.e., PMI
EM) may play an important factor for survival. Since there was only a weak correlation between PMI
EM and PMRA
EM in this study (
r=.28), it is presumed that muscle mass and quality may be mutually exclusive.
Several trials have demonstrated that early intervention has the potential to delay or prevent myosteatosis [
13]. In people at risk for developing sarcopenia-related disorders, exercise may improve muscle quality by increasing the degree of MRA [
10]. In addition, in the case of the elderly, when resistance exercise is resumed, the infiltration of muscle fat decreases [
25]. Because there are no reports of the role of exercise in improving the prognosis of cancer patients with myosteatosis, studies on the efficacy of exercise training as a predictor of survival in these patients are needed.
The ROC curve is a tool for demonstrating the sensitivity of a continuous variable to 1-specificity for all possible values of a threshold [
26], and classification accuracy is most often expressed using the AUC [
26]. Because disease outcomes are time-dependent in many occasions, the time-dependent ROC curves of the models were used in this study to characterize the time-varying prognostic performance [
27]. In this study, the iAUC of Model 1 for OS and DFS were significantly greater than that of Model 2 or Model 3. In addition, using the AUC (t)s of models over a 10-year period, Model 1 was higher than Model 2 or Model 3 in survival prediction. In addition to iAUC, the c-index is used to measure the discriminative ability of the model, with a larger c-index indicating a more accurate prognostic estimate [
24]. In the current study, c-index of Model 1 was higher than that of Model 2 or Model 3. Overall, compared to the other nested models, the full model (i.e., Model 1) showed higher c-index, iAUC, 36-month AUC, and 60-month AUC, highlighting the prognostic importance of PMRA
EM in Model 1.
Therefore, in this study, Model 1 was used to construct nomograms to accurately predict 3-year and 5-year OS and DFS. The established nomogram was internally validated using a calibration curve. In particular, patients with low PMRAEM scored approximately 50 points on the monogram, highlighting their clinical value as a predictor of survival. However, external validation is essential before applying Model 1 as a survival predictor in clinical practice. If this model is proven to accurately predict recurrence and survival, it could provide clinicians with useful preoperative information to improve surgical outcomes.
The strengths of the present study are as follows: First, to the best of our knowledge, this is the first report on the value of PMIEM and PMRAEM as determinants of survival in patients with stage I to III gastric cancer. Using multivariate Cox regression analysis, we found that PMRAEM rather than PMIEM appears a determinant of OS and DFS. Second, in this study, PMRAEM along with age, TNM stage, perineural invasion, and serum albumin level were significant covariates for both OS and DFS and constituted Model 1. Both iAUC and c-index of Model 1 had better discriminatory powers of survival than those of the nested models (i.e., Model 2 or Model 3). The result was internally validated using a resampling technique. Third, a nomogram was constructed using Model 1, and verification using a calibration curve confirmed that the predicted survival rate was almost identical to the actual survival rate. Therefore, our findings could provide clinicians with useful preoperative information to improve surgical outcomes. Fourth, in this study, a CT image was extracted by a musculoskeletal radiologist, and one nurse trained in image analysis analyzed the extracted images using a semi-automatic software program. For consistency, the final image file containing the segmentation was double checked by the radiologist. Finally, the results of this study could be the basis for the beginning of the following studies to evaluate the effectiveness of rehabilitation therapy on the ES/MF muscles to improve long-term prognosis after gastrectomy.
However, this study has some limitations, so caution is needed in interpreting the results. First, since this study was conducted retrospectively, data omissions are inevitable, and this fact may have affected the results of this study. Second, since this study was conducted retrospectively, it was impossible to evaluate nutritional status of patients in detail except for body mass index and serum albumin levels, making it difficult to study the correlation between nutritional status and PMRAEM. Finally, although we controlled for random errors and potential biases and performed internal validation, the absence of external validation was another limitation of this study.



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement1
Supplement1 Print
Print