Effect of Environmental Enrichment on Cognitive Impairment-induced by Ethanol Exposure in Adolescent Rat
Article information
Abstract
Purpose:
Adolescents who experienced the alcohol consumption have gradually increased. Adolescence is a critical period of the neural plasticity in the brain. Neural plasticity is mediated by neurotrophins and has an impact on cognitive function. Environmental enrichment ameliorates the cognitive function and increases neurotrophins. Thus, we investigated the neuroprotective effect of environmental enrichment on ethanol induced cognitive impairment in adolescent rats.
Methods:
The ethanol groups and the controls groups were injected with ethanol (0.5g/kg) and phosphate buffered saline, respectively, through intraperitoneal from 28th day of birth for 11 days. The environmental enrichment groups were provided larger cages containing toys than the standard cage. Passive avoidance test and Y-maze test were performed to evaluate the spatial memory.
Results:
Environmental enrichment+ethanol group showed higher alterations than the standard environment+ethanol group in Y-maze test (p<.05). In hippocampus, The environmental enrichment+ethanol group showed significantly higher level of the number of c-fos positive celsl and density of tropomyosin receptors kinase B receptor than the standard environment+ethanol group (p<.05).
Conclusion:
So, we suggested that the environmental enrichment played a role as a prophylaxis for prevention of memory impairment induced by ethanol exposure in adolescence.
INTRODUCTION
1. Research Necessity
Adolescence is a crucial and transition period from childhood to adulthood. Adolescence experience changes in physical and mental capabilities [1]. In physiological maturation, secondary sex-characteristics are appeared by hormonal changes and body size is increased. Meanwhile, adolescence is emotional sensitive period so emotional response is delicate, flexible [2,3]. Thus, the adolescents appear prominent tendencies toward risk-taking, sensation-seeking, and reckless behaviors (e.g., experimentation with alcohol and smoking) [3,4].
National survey on drug use and health (NSDUH) [6] reported 11.6% of adolescent aged 12 to 17 had experiences of alcohol use. Furthermore, binge drinking and heavy drinking rates were reported 6.2% and 1.2%, respectively. Alcohol use during adolescence is more harmful in cognitive function than the adulthood because cortico-limbic regions (e.g., hippocampus) are developed through neuronal plasticity during in this period [1,7]. In alcohol used adolescence, hippocampal volume as well as verbal and learning and memory function were diminished significantly [8,9]. In adolescence animal model, intermittent ethanol exposure caused inflammatory damage in prefrontal cortex and hippocampus and impairment of short-term memory, long-term memory and motor function [10].
Hippocampus plays important role in the learning and memory. Hippocampal neuroplasticity is regulated by neurotrophic factors, such as brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), neutrophin-3 (NT-3). BDNF is the most abundant factor in hippocampus [11,12]. BDNF is a major regulator of synaptic transmission, play a role in plasticity such as long-term potentiation (LTP) [12] and regulated by cAMP response element-binding (CREB), c-fos that are known as transcription factors [13,14].
Environmental enrichment (EE) is housing condition that promotes enhanced sensory, cognitive, motor, social stimulation compare to the standard housing condition [14]. EE exposure has been reported to increase brain neurotrophins. Especially, BDNF level in hippocampus was significantly higher in EE group than the standard environment group [11,14].
Neuroprotective effect of EE in cognitive function have been reported in various neuronal disease such as Alzheimer’s disease [15,16]; Parkinson’s disease [17]; Attention Deficit Hyperactivity Disorder (ADHD) [18]. However, there is little known about the effect of EE in ethanol-induced cognitive impairment. In the present study, we conducted to confirm the neuroprotective effect of EE on ethanol-induced cognitive impairment in adolescence.
2. Research Purpose
The purpose was to investigate the effect of EE on cognitive impairment in ethanol-induced cognitive impairment in adolescent rats. The specific purposes in the study are as follows:
1) Evaluates the effect of EE on spatial memory using behavior tests in ethanol exposed adolescent rats.
2) Evaluates the effect of EE on neuroplasticity mediating factor levels in hippocampus in ethanol exposed adolescent rats.
MATERIALS and METHODS
1. Experiment Design
The number of animals in each group was as follows: (1) SE+PBS group (n=12); (2) SE + EtOH group (n=12); (3) EE+PBS group (n=16); (4) EE+EtOH group (n=16)(Figure 1). EE groups were provided the large cage (75x45x25cm) containing tunnels, platforms, toys (e.g., toy balls, colorful plastic or wooden blocks), running wheels to stimulate learning, memory, sensory and motor function, and social interactions. We replaced old toys with new toys once a week. And new toys were selected with different shape or size or color or texture from old toys because of frequent changes of toys facilitate neural activity [20]. On the 28th days of birth, the ethanol was administered by intraperitoneal injection for 11days, dose of 0.5g/kg. Meanwhile, the control groups were received isovolumetric phosphate buffered saline (PBS) injections to maintain equivalent experiment conditions. The experiment was performed in accordance with the animal experimentation of ‘K’ University in Korea, and NIH guide for the care and use of laboratory animals.
2. Animals
The four pregnant rats of the Sprague-Dawley (Orient Bio, Seongnam, Korea) were obtained after timed mating. At two days before delivery, two of pregnant rats were moved into EE cage (n=2) and the others were remained in standard environment (SE) (n=2) randomly. All of animals were housed in a temperature (23±2°C), humidity (50-55%), light (12h light-dark cycle)-controlled environment with access to food and water ad libitum
3. Methods
1) Y-maze test
Y-maze test is used to assesses short-term spatial memory in rodents. The maze was made of wood and 3 arms were positioned at equal angles. The serial arm entries were recorded for 5 min. Passing by half of the arm was recorded as entry. Alternation was considered as non-overlapping triplet set entries. The test was executed on the 39th day of birth. Alteration rates were calculated with followed formula, ‘Percentage of alternation (%) = (alternation / total number of arm entries- 2) × 100’. Closer to 100% alteration percentage of rat it means spatial memory function is better than lower alteration rat.
2) Passive-avoidance test
In rodent, passive avoidance test is a popular and easily used behavior test measuring long-term memory related to hippocampus function [20]. The apparatus consisted of light and dark acryl compartment. These two compartments were separated with a guillotine door. During the acquisition trial, rats were placed in the light compartment for 10s of habituation and guillotine door was opened. Immediately after rat entered the dark compartment, door was closed meanwhile electrical shock delivered (0.75mA, 50Hz) for 2 s through stainless steel floor. 24h later, the retention test was proceeded. The latency time until entering the dark compartment was measured without electrical shock. The maximum latency time was 600s. The test was executed on the 39th day of birth after Y-maze test. The latency time positively correlates with long-term memory [20].
3) Immunohistochemistry
Using immunohistochemical techniques, we evaluated c-fos and CREB expression in hippocampus. c-fos and CREB are transcription factors that bind to DNA and regulate BDNF expression. Animals were sacrificed under anesthesia. Then, animals were perfused and immersed with ice-cold PBS followed by 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4). Fixed brain tissues were storage in the sucrose solution for 3days on 4°C. Serial coronal sections of brains were cut into 40um thick with a microtome (CM3050S, Leica, Nussloch, Germany) and were immersed in storing solution. The brain sections were incubated in the primary antibody c-fos (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and phospho-CREB (1:1000; Millipore, Temecula, CA, USA) for 24h. Then sections were washed with PBS and incubated in a biotinylated anti-rabbit secondary antibody (1:400; Vector Laboratories, Brulingame, CA, USA) for 1hr. Next, the sections were incubated in avidin–biotin–peroxidase complex (Vector Laboratories, Brulingame, CA, USA) for 1hr. For the developing of color, 0.4% diaminobenzidine (Vector Laboratories, Brulingame, CA, USA) was used as the chromogen. The number of c-fos and phospho-CREB positive cells in the hippocampus was measured with image analysis system (ImagePro Plus, Media Cyberbetics Inc., Silver Spring, MD, USA).
4) Western blot
The BDNF and trkB protein levels in hippocampus were evaluated by western blot. Increased expression of BDNF and trkB indicate the hippocampal synaptic plasticity and LTP. Hippocampal tissues were homogenized in a lysis buffer (iNtRON Biosystems, Seongnam, Korea). Proteins were resolved by 10% sodium dodecyl sulfate (SDS) polyacrylamide gels and electro-transferred onto a nitrocellulose membrane. The membranes were blocked with 5% skim milk in Tris-buffered saline, 0.1% Tween 20 (TBST). Membranes were then incubated with anti-BDNF (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-trkB (1:1000; Cell Signaling Technology, Beverly, MA, USA) for a day. Membranes were incubated for 2h with horseradish peroxidase conjugated secondary antibody (anti-rabbit antibody, 1:5000, Molecular Probes, Eugene, OR, USA) in 5% skim milk in TBST and developed using enhanced chemiluminescence western blotting detection system (SuperSignal West Pico, Thermo Scientific, Rockford, IL, USA). The relative optical densities of the bands visible on X-ray film were scanned and digitized by Image J 1.42q software (NIH, Bethesda, MD, USA). All relative intensities were normalized to β-actin expression in the same sample.
4. Statistical Analysis
Statistical analyses were performed by SPSS 22.0. All data were presented as mean±SE. Statistical comparison processed with one-way ANOVA, followed by Tukey post-hoc test for comparisons among groups. The p<.05 was used as the criterion for statistical significance.
RESULT
1. Environmental enrichment protected memory impairment induced by ethanol exposure
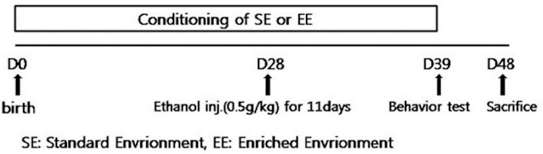
The Y-maze and passive avoidance test were employed to evaluate the memory function of all the rats. The result of Y-maze test showed that EE+EtOH group (77.10±9.30) had significantly higher percentage of alternation compare to the SE+EtOH group (60.28±11.61) (p<.05) (Figure 2A). The passive avoidance test showed a difference without significance between SE+EtOH group (25.00±5.47) and EE+EtOH group (79.71±20.63) (Figure 2B).
Improvement of cognitive function by environmental enrichment in ethanol exposure induced cognitive impairment adolescent rat on behavior test. Result of Y-maze test is shown in (a). Result of Passive avoidance test is shown in (b). Data are expressed as mean±SE. *p<.05 compared to the SE+EtOH group.
SE, Standard Environment; EE, Enriched Environment; PBS, Phosphate Buffered Saline; EtOH, Ethanol.
2. Environmental enrichment enhanced c-fos expression in hippocampus
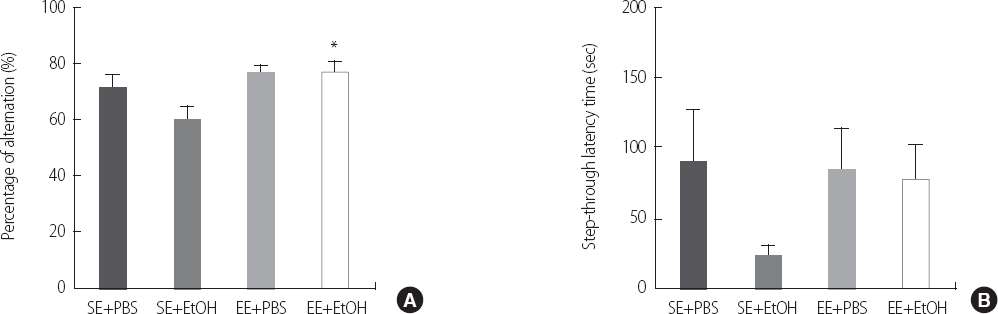
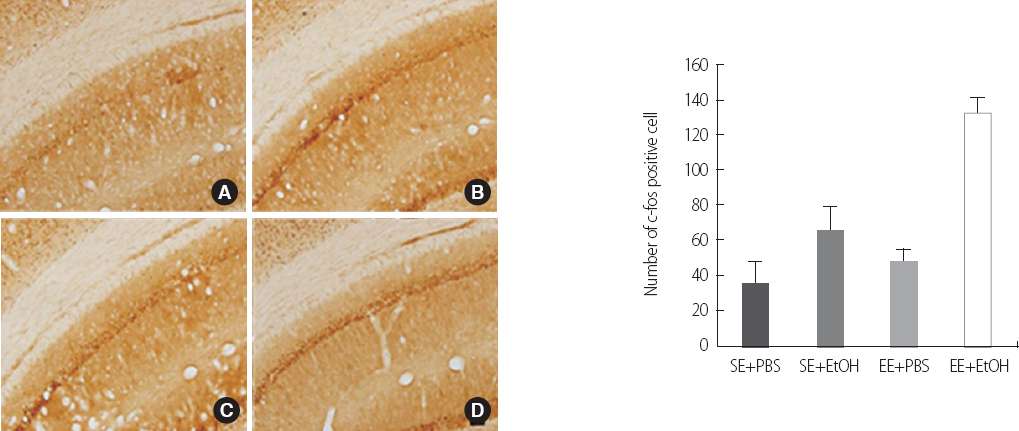
To investigate the transcriptional factor affecting the expression of BDNF, we performed the immunostaining of c-fos and phospho-CREB. The number of c-fos positive cell in the EE+EtOH (131.50±15.89) group was significantly increased than the SE+EtOH group (65.17±32.95) (p<.05) (Figure 3). However, the phospho-CREB positive cells in SE+EtOH (61.33±8.24) group was a lot more than the EE+EtOH (58.67±6.00) (Figure 4).
Immunostaining of c-fos in hippocampus CA1 in ethanol exposure induced cognitive imparement adolescent rat. Sections were stained for c-Fos-positive cells (reddish brown). Scale bar represents 100 um. (A) SE+PBS, (B) SE+EtOH, (C) EE+PBS and (D) EE+EtOH group. Data are expressed as mean±SE. *p<.05 compared to the SE+EtOH group.
SE, Standard Environment; EE, Enriched Environment; PBS, Phosphate Buffered Saline; EtOH, Ethanol.
Immunohistochemistry of phospho-CREB in hippocampus CA1 in ethanol exposure induced cognitive impairment adolescent rat. Scale bar represents 100 um. (A) SE+PBS, (B) SE+EtOH, (C) EE+PBS and (D) EE+EtOH group. Data are expressed as mean±SE. *p<.05 compared to the SE+EtOH group.
p-CREB, phosphor-CREB; SE, Standard Environment; EE, Enriched Environment; PBS, Phosphate Buffered Saline; EtOH, Ethanol.
3. Environmental enrichment increased trkB expression in hippocampus
Neural plasticity is influenced by several neurotrophic factors, one of the factors is BDNF.
In western blotting, the expression of BDNF showed difference without significance between EE+EtOH group (.55±.22) and SE+EtOH group (.42±.15) (Figure 5-A). The expression of trkB that is highest affinity to the binding the BDNF showed that the EE+EtOH group (.80±.12) had significantly higher density compared to the SE+EtOH group (.42±.18) (Figure 5-B) (p<.05).
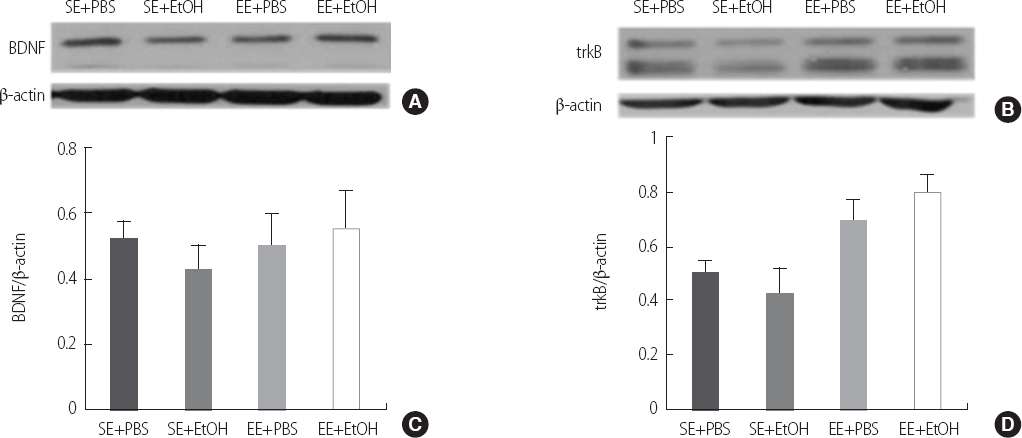
The synaptic plasticity in ethanol exposure induced cognitive impairment adolescent rat of by western blotting. Representative western blot of BDNF is shown in (a). BDNF densitometry analyses are shown in (b). Representative western blot of trkB is shown in (c). trkB densitometry analyses are shown in (d). Data are expressed as mean±SE. *p<.05 compared to the SE+EtOH group.
SE, Standard Environment; EE, Enriched Environment; PBS, Phosphate Buffered Saline; EtOH, Ethanol.
DISCUSSION
Our study was designed to confirm the positive effect of EE on cognitive impairment induced by ethanol in adolescent rats. In the present study, the c-fos positive cells were significantly increased in the EE+EtOH group compared to the SE+EtOH group. Also, the trkB protein levels were increased in the EE+EtOH group compared to the SE+EtOH group, representing increased synaptic plasticity in hippocampus. Also, these neurobiological changes were reflected in behavioral memory function test. These results demonstrated that EE protected the cognitive impairment induced by ethanol exposure in adolescence.
The results of Y-maze and passive avoidance test presented that EE protected ethanol induced memory impairment. Particularly, in the EE+EtOH group, spatial memory showed a significant difference compared to that of the SE+EtOH.
The number of c-fos positive cells in the hippocampus was significantly increased in EE+EtOH group compared to the SE+EtOH. The c-fos is an immediate early gene family of transcription factor as a marker of stimulus-induced change in the central nervous system [21]. And many studies have shown that c-fos might be used a marker of neuronal activity and closely involved in the formation of long-term memory [21,22]. Also, Zhang et al. [14] reported that c-fos mediated neuronal excitability and survival in kainic acid treatment. Thus, these previous results were consistent with our result of study. The CREB is a transcription factor and plays a role in learning and memory through the regulation of synaptic plasticity. However, the number of phospho-CREB positive cells in the hippocampus presented no differences among groups.
BDNF enhances glutamatergic synaptic transmission and phosphorylation of the subunits of N-methyl-D-aspartate (NMDA) receptors resulting in lasting LTP in the hippocampus. And the effects of BDNF in LTP are mediated by TrkB receptor [23]. Impairment of LTP in the BDNF mutant mice with intact TrkB was ameliorated by the exogenous BDNF [24]. In our result, the level of BDNF showed no differences among groups. However its receptor, trkB level was increased in the EE+EtOH group compared to the SE+EtOH group. So, we believe that elevated trkB level enhanced BDNF downstream and protected memory function.
In this study, we designed to investigate the influence of EE in ethanol exposed adolescent using behavioral and molecular research methods. As a result, we confirmed that the EE during adolescent period plays a role as prophylaxis from cognitive impairment in adolescence. So, we suggest that the quality of living environment is important to cognitive function during infancy to adolescence. Also, this finding would be contribute to evidence based nursing in environmental nursing intervention.
CONCLUSION
The results of our study suggest that the EE prevented the memory impairment and enhanced synaptic plasticity in ethanol exposed adolescent rat.